A research study consists of several steps: find a topic, form a hypothesis, collect data, analyze data, and create conclusions. It is hard work to conduct research and thus, it is normal for researchers to feel protective over their procedures. Though it is ideal to be in control of every aspect, unexpected situations may arise. When the study departs from the original plan or circumstances arise that change the course of a study, it is common for researchers to feel frustration or concern. In these cases, the IRB is here to help researchers process and manage these events.
Protocol Deviations
A protocol deviation is a departure from the research design that has not been approved by the IRB. This can affect any approved procedures or information in the protocol, consent form, assent form, recruitment materials. These deviations can be intentional, expected, or unplanned. Protocol deviations manifest through a wide range of events and carry varying levels of risk, some posing minimal risk to participants, while others may have serious repercussions and rise to the level of an adverse event. Any change or non-compliance with an IRB protocol that poses minimal risk to the subject typically falls under the category of a protocol deviation. Some examples of protocol deviations from least to most risky include:
- A publicly available online survey generates more interest than expected. More participants than initially anticipated respond, exceeding the approved enrollment without increasing risks to participants.
- A researcher’s CITI certification expires, and the researcher continues to conduct research activities that they had been approved to conduct prior to updating their CITI certification.
- Principal Investigator (PI) decides to use a different survey platform for data collection than what was approved in the protocol.
- Participants are kicked out in the middle of the online surveys due to wifi problems.
- A member of the research team fails to properly obtain informed consent before carrying out study procedures
In all cases, any changes or events that affected the methods or procedures must be reported. PI should report a protocol deviation or adverse event to the IRB as soon as possible, as additional risks could be incurred during the reporting time.
Adverse Events
An adverse event is when the participant experiences physical or psychological harm or is put at risk during the research procedure, whether or not it is directly caused by the study. Adverse events are typically unexpected and unintentional, and they are much more difficult to avoid than protocol deviations. Adverse events may range from mild to severe, unexpected to expected, and unrelated to definitely related. The most serious adverse events may be life-threatening. Cases of adverse events include:
- A natural disaster damages the room used to collect data.
- Participant research data is hacked and leaked.
- Participant is hit by a car on their way to or from the research site and is transported to the hospital.
- A researcher makes an error in dosing or dispensing a study medication, causing participants to overdose and require medical attention.
- A participant with a history of suicidality participates in a study containing questions regarding the participant’s personal experiences with suicide. The participant completes the study and appears stable upon leaving the research site. The participant’s family later reports to the research team that the participant died by suicide shortly after their involvement with the research study.
- A participant in a clinical trial for stage 4 cancer passes away.
For more information, please check out our blog on When to Submit an Adverse Event.
Hierarchy of Controls and Identification of Hazards
Though not all adverse events are life threatening, it is best for the researcher to prepare for them. While adverse events can happen in online studies, they more commonly occur when research studies are conducted on-site and in-person. In the case there is a risk that can be easily controlled by the researcher, the researcher may be able to prevent the problem from occuring or get an expert researcher to help the participant.
The Hierarchy of Controls lists methods that are most effective to least effective in preventing an adverse event.
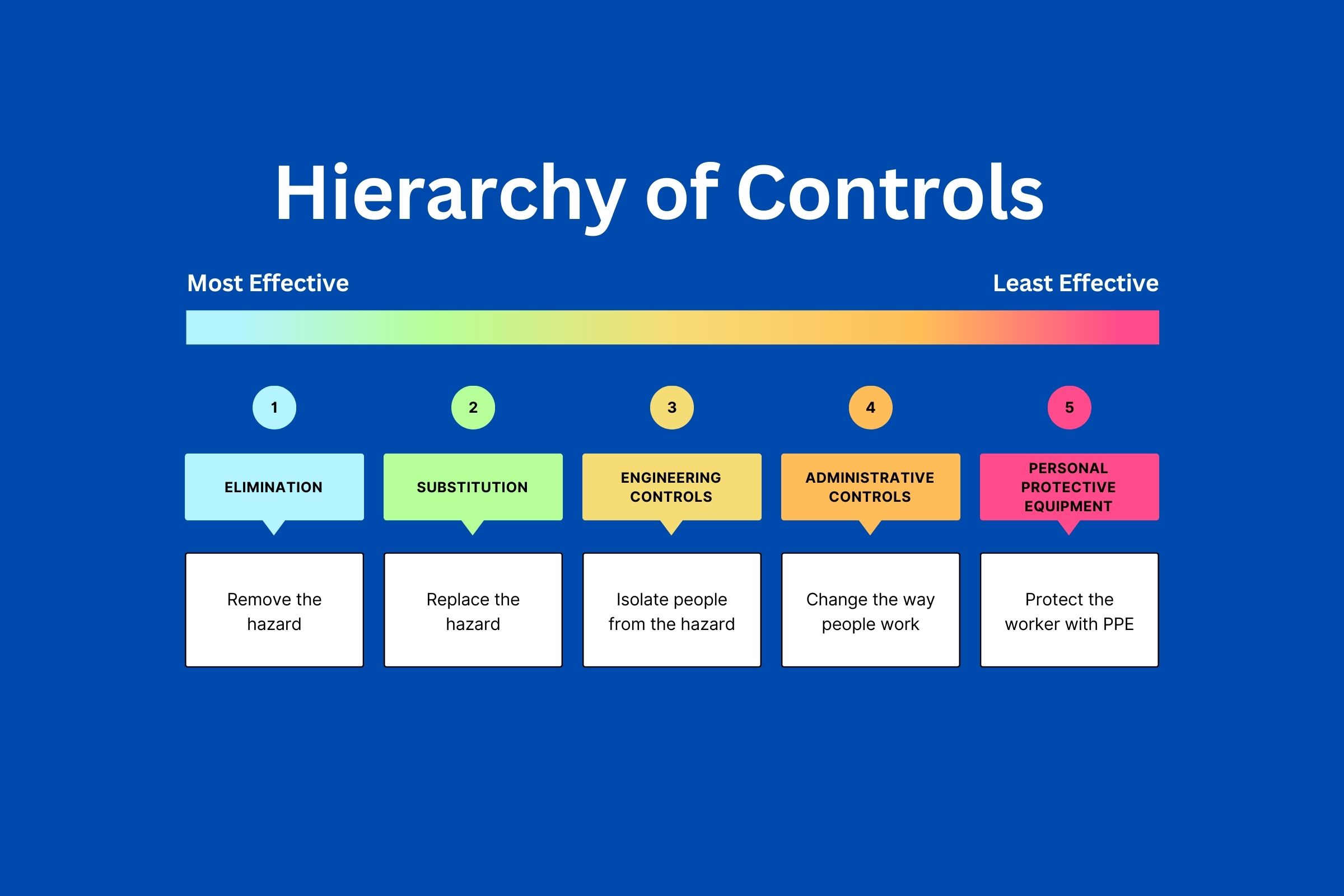
Hierarchy of Controls Infographic - Accessible Version
However, in all cases, the researcher should only manage the risk if they have control over the hazard. Some prevention controls require the researcher to help the participants, such as giving them places to rest after getting their blood drawn; but if the hazard is unexpected, the researcher should protect themselves and trust professionals to manage the situation. If working with vulnerable patients, it is recommended that there is more than one assistant on site just in case.
On Campus Resources for Reporting
There are many on campus resources that can help the researcher and student participants when the event occurs.
- Columbia Health Services to know more about health services offered and schedule an appointment.
- Counseling and Psychological Services to create an appointment with a counselor. Researchers can also contact 212-854-2878 for immediate assistance.
- Alice! Health Promotion to provide sexual health peer counseling to students of all gender identities and orientations
- For any emergency situations during any point of the research process, please call 911. For on campus emergencies, please contact the Department of Public Health and Safety (DPS) (212) 678-3333.
Most importantly, researchers must not conduct actions that risk their own safety and well-being. There is always a strong need to help, but the safest way to protect yourself is calling professionals to help you.
Updating Protocols
Like protocol deviations, adverse events affect the approved protocol and must be reported to the IRB as soon as possible. Though it may seem tedious to update the protocol, doing so informs the IRB about what happened and allows them to investigate the issues that led to the event. That way, the IRB can identify risks and find solutions to protect the researcher and participants if the same procedures are used in future studies. Additionally, reporting events in a timely manner will allow the researchers to continue their research quickly and ethically. Some researchers may feel apprehensive about reporting a protocol deviation or adverse event, however, these reports ensure that TC IRB is able to record, evaluate and correct any issues. This is a required procedure, as not reporting a protocol deviation or adverse event constitutes non-compliance. While not all events are foreseeable, the IRB is here to support you through each event.
